ACIDS, BASES AND SALTS
More MCQs of this Chapter
Playlist of Class 10 Science MCQs
_____ are sour in taste and change the colour of blue litmus to red.
(a) acid
(b) base
(c) salt
(d) neutral
________ are bitter and change the colour of the red litmus to blue
(a) acid
(b) base
(c) salt
(d) neutral
________are corrosive in nature.
(a) acid
(b) base
(c) salt
(d) neutral
Litmus solution is a purple dye, which is extracted from lichen, a plant belonging to the division Thallophyta, and is commonly used as
an _________.
(a) Indicator
(b) lactic acid
(c) turmeric
(d) base
Materials like red cabbage leaves, turmeric, coloured petals of some flowers such as Hydrangea, Petunia and Geranium are __________
(a) Natural indicator
(b) Synthetic Indicator
(c) Olfactory indicator
(d) Universal indicator
Which substance play an important role in our struggle for independence?
(a) HCl
(b) NaCl
(c) CaCO3
(d) None of these
Water is neutral because ______
(a) It has H- ions
(b) it has equal no. of H+ ions and OH- ions
(c) It has OH- ions
(d) it is colourless
All the chemical compounds can be classified on the basis of their chemical properties as acids bases and _______
(a) acid
(b) base
(c) salt
(d) none of these
What is the nature of litmus solution?
(a) acidic solution
(b) base solution
(c) salt solution
(d) neutral solution
Acids can be classified as organic acids and _________ acids
(a) mineral
(b) Dilute
(c) Mix
(d) Natural
Which of the chemical substance used for manufacturing cold drink
(a) nitric acid
(b) Hydrochloric acid
(c) sulphuric acid
(d) none of these
Hint : Carbonic acid
which type of acid present in curd?
(a) lactic acid
(b) hydrochloric acids
(c) formic acids
(d) malic acid
What is the pH range of our body?
(a) 7.0 – 7.8
(b) 6.0 – 8.0
(c) 1.5 – 3.4
(d) 7.2 – 8.4
which type of acid present in Stomach?
(a) lactic acid
(b) hydrochloric acids
(c) formic acids
(d) malic acid
Which of the following is a weak base?
(a) Sodium hydroxide
(b) potassium hydroxide
(c) aluminium hydroxide
(d) lithium hydroxide
Which of the following acid is used as a preservative in making pickles?
(a) lactic acid
(b) hydrochloric acids
(c) formic acids
(d) acetic acid
Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
which type of acid present in Ant bee?
(a) lactic acid
(b) hydrochloric acids
(c) formic acids
(d) malic acid
which type of acid present in vinegar?
(a) lactic acid
(b) hydrochloric acids
(c) Acetic acids
(d) malic acid
which type of acid present in Apple?
(a) lactic acid
(b) citric acids
(c) tannic acids
(d) malic acid
which type of acid present in Tea?
(a) lactic acid
(b) citric acids
(c) tannic acids
(d) malic acid
which type of acid present in Lemon?
(a) lactic acid
(b) citric acids
(c) tannic acids
(d) malic acid
H2SO4 is an ________ acid
(a) mineral
(b) citric acids
(c) tannic acids
(d) malic acid
Tooth enamel is made up of _________
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
When acid react with water release __________ions.
(a) Pb-
(b) Oh-
(c) H+
(d) C+
___________ are substances that change colour when they are added to acidic or alkaline solutions.
(a)indicator
(b) lactic acid
(c)turmeric
(d) base
__________ indicators such as methyl orange(red in acid and yellow in base) and phenolphthalein (colourless in acid and Pink in basic) to test for acids and bases.
(a) Natural indicator
(b) Synthetic Indicator
(c) Olfactory indicator
(d)Universal indicator
Onion is___________ indicator
(a)Natural indicator
(b)Synthetic Indicator
(c)Olfactory indicator
(d) Universal indicator
When onion juice reacts with ___________, No change in smell of onion.
(a) acid
(b) base
(c) salt
(d) neutral
Vanilla essence, clove oil is ______________
(a) Natural indicator
(b) Synthetic Indicator
(c) Olfactory indicator
(d) Universal indicator
Acid + Metal —>_____ + Hydrogen gas
(a) acid
(b) base
(c) salt
(d) Metal hydroxide

When copper (Cu) reacts with hydrochloric acid (HCl). What will happen?
(a) React vigorously
(b) No reaction
(c) React Strongly
(d) React slowly
Metal carbonate/Metal hydrogen carbonate + Acid –>Salt + ________ + Water
(a) Carbon dioxide
(b) Oxygen
(c)Nitrogen
(d) Metal hydroxide
Carbonic acid break down __________
(a) HCl
(b) Na2CO3
(c) Nitrogen
(d) Ca(OH)
Maximum metallic oxide is _____ in nature
(a) acid
(b) base
(c) salt
(d) Metal hydroxide
Note: (zinc oxide and aluminium oxide are amphoteric in nature)
Metal oxide + Acid Salt + __________
(a) acid
(b) base
(c) salt
(d) water
Copper oxide(base)+ Hydrochloric acid(acid)–> copper chloride (______) and water
In the Above Reaction Copper chloride is ______
(a) acid
(b) base
(c) salt
(d) water
Base + Acid –>Salt + Water
Generally metal hydroxide is ___________ in nature
(a) acid
(b) base
(c) salt
(d) water
___ produce (OH−) ions when dissolved in water.
(a) acid
(b) base
(c) salt
(d) water
base + Metal –>Salt + ____________
(a) Hydrogen
(b) Oxygen
(c) Nitrogen
(d) Chlorine
Hint: 2NaOH + Zn ⇨ Na2ZnO2 + H2
Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal.
Bases generate hydroxide (OH–) ions in water. Bases which are soluble in _______ are called alkalis.
(a) Hydrogen
(b) Water
(c) Nitrogen
(d) Chlorine
Nonmetallic oxide is ______ in nature
(a) acid
(b) base
(c) salt
(d) water
CO2 _________ + Nonmetallic oxide—>Salt + water
(a) acid
(b) base
(c) salt
(d) water
Hint : 2NaOH+CO2⟶Na2CO3+H2O
Mixing an acid or base with water results in decrease in the concentration of ions (H3O+/OH–) per unit volume. Such a process is called ____________ and the acid or the base is said to be diluted.
(a) acidification
(b) Dilution
(c) Indication
(d) Filtration
The process of dissolving an acid or a base in water is a highly ___________ one.
(a) Endothermic
(b) Exothermic
(c) Filtration
(d) None of these
![]()
![]()
(a) Hydrogen
(b) Carbon dioxide
(c) Nitrogen
(d) Chlorine
Chemical formula of common salt ___________
(a) H2SO4
(b) NaCl
(c) HCl
(d) CaO
HCl + NaOH —> NaCl + __________
(a) Hydrogen
(b) Water
(c) Nitrogen
(d) Chlorine
Sodium Bicarbonate is a chemical Name of _____________
(a) common salt
(b) Plaster of Paris
(c) baking soda
(d) None of these
Hint : NaHCO3
_____________ is an aqueous solution of sodium chloride
(a) common salt
(b) Brine
(c) Lime stone
(d) None of these

(a) Ammonia
(b) Slaked lime
(c) Lime stone
(d) None of these
Hint: NH3

(a) Hydrogen
(b) carbon dioxide
(c) Nitrogen
(d) Chlorine
_______ is also used in fire extinguishers.
(a) Ammonia
(b) Slaked lime
(c) Lime stone
(d) None of these
Hint: Sodium Bicarbonate
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide/caustic soda. The process is called the ______process
(a) Ammonification
(b) acid-alkali
(c) chlor-alkali
(d) None of these
Note:because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
![]()

Chlorine gas is given off at the _______, and hydrogen gas at the_____. Sodium hydroxide solution is formed near the cathode.
(a) cathode, anode
(b) anode, cathode
(c) chlor-alkali, anode
(d) None of these
_________________ is a chemical name of Bleaching powder
(a) Calcium oxide
(b) calcium bicarbonate
(c) calcium hypochlorite
(d) None of these
Hint : calcium oxychloride
chemical formula of bleaching powder
(a) CaO
(b) CaOCo3
(c) CaOCl2
(d) None of these
The ___________ is commonly used in the kitchen for making tasty crispy pakoras, etc. Sometimes it is added for faster cooking.
(a) Ammonia
(b) Slaked lime
(c) Lime stone
(d) baking soda
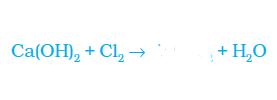
(a) CaO
(b) CaOCo3
(c) CaOCl2
(d) None of these
Washing soda is synthesized through ___________ process
(a) carbonic
(b) solvay’s
(c) aristic process
(d) None of these
Chemical formula of washing soda
(a) Na2CO3
(b) Na2CO3.10H2O
(c) Na2CO3.5H2O
(d) None of these
On heating gypsum at ______ K, it loses water molecules and becomes calcium sulphate hemihydrate
(a) 273
(b) 373
(c) 353
(d) 100
Water of ___________ is the fixed number of water molecules present in one formula unit of a salt.
(a) neutral
(b) concentrated
(c) crystallization
(d) None of these
![]() is known as _______________
is known as _______________
(a) gypsum
(b) Plaster of Paris
(c) crystal of acid
(d) None of these
The atmosphere of venus is made up of thick white and yellowish clouds of _________
(a) gypsum
(b) sulphuricacid.
(c)crystal of acid
(d) carbon dioxide
A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
A solution reacts with crushed-egg shells to give a gas that turns lime water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
10 mL of a solution of NaOH is found to be completely neutralized by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralize
it will be_______
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
This Questions is not MCQ
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is
(a) Neutral
(b) Strongly alkaline
(c) Strongly acidic
(d) Weakly acidic
(e) Weakly alkaline
At what temperature is gypsum heated to form Plaster of Paris?
(a) 125°C
(b) 100°C
(c) 105°C
(d) 120°C
Assertion: HCl change the colour of blue litmus to red.
Reason: HCl is acid.
Assertion: Phenophtalein gives pink colour in basic solution
Reason: Phenophtalein is a natural indicator
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: Plaster of paris is used by doctor to setting fractured bones
Reason: When plaster of paris is mixed with water and applied around the fractured limbs its set into a hard mass
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: baking soda creates acidity in the stomach.
Reason: baking soda is alkaline
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: Weak acids have low electrical conductivity
Reason: Strong acids and weak acids have equal concentration of hydrogen ions in their solution
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: HCL gas does not change the colour of dry blue litmus paper
Reason: HCL gas dissolves in the water present in wet litmus paper tov form h + ions
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: Ammonia solution is an alkali
Reason: Ammonia solution turns red litmus paper to blue
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: Sodium hydroxide reacts with zinc to produce hydrogen gas
Reason: acids react with active metals to produce hydrogen gas
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Assertion: H2CO3 is a strong acid
Reason: A strong acid this dissociates completely or almost completely in a water
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.
Hint: H2CO3(weak acid)
Carbonic acid
Assertion: if the pH inside the mouth decrease below 5.5 the decay of tooth enamel begins
Reason: the bacteria present in mouth degrades the sugar and leftover food particles and produce acids that remains in the mouth after eating
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are False.
(d) Both A and R are true and R is the correct explanation of A.
(e) Both A and R are true but R is not the correct explanation of A.